
Secondary electro-spray ionization (SESI)
Secondary electro-spray ionization (SESI) is an ambient ionization technique for the analysis of trace concentrations of vapors, where a nano-electrospray produces charging agents that collide with the analyte molecules directly in gas-phase. In the subsequent reaction, the charge is transferred and vapors get ionized, most molecules get protonated (in positive mode) and deprotonated (in negative mode). SESI works in combination with mass spectrometry or ion-mobility spectrometry.
Low volatility species: their importance.
High volatile species can be easily detected at very low concentrations, but things get more complicated with low volatility molecules…
Low volatility species (such as some endogenous or exogenous metabolites, proteins, viral particles, drug metabolites…) tend to be more relevant from a biological standpoint. These larger molecules carry more information and are more process-specific, but they are often very diluted because they have a low vapor pressure.
SESI is optimized to ionize large and low volatility molecules in the gas phase.
Why SESI is ideal for ionizing low volatility molecules:
- Because ionizing at atmospheric pressure improves limits of detection:
Turbulent losses and condensation losses are eliminated, which means more molecules reach the ionization area. Since molecules are ionized before they reach the adiabatic expansion and cooling, region process (instead of at the entrance of the ionizer as it happens in low-pressure operating systems), ions are heated by electric fields and focused.
The ionization reaction is much faster, which means better ionization efficiency. The velocity scales with (1) the concentration of the vapors and (2) the concentration of the charging ions, both of them go with the pressure in the ionizer. At room pressure (10^3 mBar), the velocity of the charge reaction is 10^6 times higher than at 1 mBar.
High performance Electrospray - MS systems are optimized to transfer and desolvate heavy ions from atmospheric pressure into their vacuum side. This challenging technical problem is already solved by currently available commercial MS systems. SSX just rides very powerful horses!
The ionization efficiency of nano-electrospray is extremely high because the concentration of charging agents near the nano-jet is extremely high
- Because the nano-electrospray provides very clean spectra:
in-source ion fragmentation and oxidation is very low because there are no high energy ions present at any point in the ionizer. Compared with other sources using plasma as a source of charging ions, SESI ions are formed from evaporating nano-droplets. The result is a lot simpler spectra, which are easier to interpret. This is particularly important for untargeted studies, like biomarker discovery.
Want to see data to support this claim?
“While using SESI we can mostly see protonated/deprotonated species in the spectra (signal-to-noise ratio (S/N) = 115) , other technologies that use high-energy ionization, as PI, often show also oxygenated species (S/N = 5).
For more information about this topic, you can read the full” Check the full peer-reviewed article here.
What is the ionization efficiency?
The ionization efficiency the key for sensitivity, which is required to detect species at very low concentration.
The ionization efficiency of each chemical species is different, and it is defined as the ratio of ions delivered to the mass spectrometer, over molecules introduced in the ionizer. It measures the percentage of the molecules introduced in the ionizer that are ionized and transferred to the mass spectrometer.
Having a high ionization efficiency is required to detect the biologically relevant molecules in the gas phase. The amount of molecules that pass from the lining of the lung into the gas phase, or from a cell culture or other objects to the headspace above it, depends on the volatility of each substance.
Low volatility species have low vapor pressures, and they are evaporated at very low concentrations, even if they are abundant in the condensed phase. As a result, the concentration of biologically relevant molecules in the gas phase is very low, so we need to have a high ionization efficiency to detect them.
WHAT AFFECTS THE IONIZATION EFFICIENCY?
The charge transfer reaction rate. In positive mode, SESI ionizes the molecules with higher proton affinity than the water clusters (the proton affinity of water in the gas phase is 697 kJ/mol). In special applications targeting known compounds, other charging solutions can be used to narrow the species that are ionized.
The reaction rate in SESI can be very high for ionizable species because the high pressure keeps the charging ions, the moisture, and the molecules very densely packed.
The actual concentration of the sample molecules in the ionization region. If not carefully designed, the sample flow required to carry the vapors to the ionizer, and the flow of gas sampled by the MS, can greatly dilute the molecules of interest, thus reducing the amount of ions produced.
The transmission of ions to the mass spectrometer. For this, the ionization region must communicate with the mass spectrometer. However, if not carefully designed, this opening can lead to gas mixing and dilution of the neutral molecules. A properly designed SESI avoids dilution while maximizing the passage of ions from the ionization region to the analyzer.
The humidity level, which has a great effect on the reaction rate.
Super SESI is optimized for ionization efficiency!
Volatilomics & Breath analysis
Super SESI can detect minute concentrations of low volatility species in real time, with molecular masses as high as 700 Da, falling in the realm of metabolomics. These molecules are naturally released by living organisms, and are commonly detected as odors, which means that they can be analyzed non-invasively. SESI, combined with High Resolution Mass Spectrometry, provides time-resolved, biologically relevant information of living systems, where the system does not need to be interfered with. This allows to seamlessly capture the time evolution of their metabolism and their response to controlled stimuli.
What is the background level?
The background level refers to the spectra that can be measured when no sample is introduced in the ionizer. An ideal analyzer should provide a signal when the sample is introduced, and no sample when no signal is introduced. However, the ionizer produces ions even when no sample is introduced:
Primary ions produced by the electrospray. This includes charging ions and other contaminants in the electro-spray liquid.
Carryover effects. Low volatility species tend to adhere in the internal walls of the ionizer. These species are then released back in the gas phase until they are fully depleted. This results in a signal that lasts longer than the actual exposure to the sample.
The background level depends on the previous history of the ionizer, and can change over time. Carryover effects are dependent, with lower volatility species having longer wash out times.
(Left) each curve is the result of extracting list of peaks and averaged intensities, ordering peak intensity from higher to lower intensity and plotting intensity vs index. This was done for each exhalation in the data-set and for the background acquired before the exhalation.
(Right) shows the mean and 1st to 9th deciles for breath and background, and the average number of peaks that rise above the 10^3 a.u threshold.
Why background levels are important:
Having a low background level is required to detect biologically relevant molecules in the gas phase.
Low volatility species tend to have strong carryover effects, which lead to high background levels that deteriorate the Limits of Detection (LoD). In the gas phase, low volatility species are present in very low concentrations, which require very high ionization efficiency. As the ionization efficiency is improved, LoD is more and more determined by background levels.
The ionization efficiency of SUPER SESI is sufficient to produce clear signals even for very low volatility species, but that doesn't mean that they can be always detected. In the high mass range of volatile compounds (200 to 700 Da), the main factor defining the LoD is the background level.
Having a well characterized background level is required to differentiate signal variations caused by the sample from other variations.
The fact that the background level can change over time because it depends on the previous history of the ionizer can pose some difficulties to analyze the data. In a long bio-marker discovery clinical study, background changes can be a serious source of confounding results.
When monitoring real time metabolic changes, the time resolution of the analysis is defined by the memory effect time of each species. Improving memory effects enables better time resolved in-vivo metabolic studies.
What affects the background level:
The background level may depend on many factors. These are some of the most important:
Exposure: a specific molecular mass may appear in the background of the ionizer only if the ionizer is exposed to it. As obvious as this may sound, it is important to bear it in mind. SUPER SESI is designed to analyze the composition of breath, and we will be breathing on it as we take it out of its box!! Another important source of contamination is the bare hand. SUPER SESI should be handled with clean gloves, not because it is dangerous to the skin, but to avoid contaminating it.
Surface available: memory effects depend on the interaction between the volatile and the internal surfaces. More surface means more memory effects. The electrode-less configuration was indeed developed to dramatically reduce the available surface. The type of surface available is also very important. All surfaces exposed to the volatile are coated with analytical grade passivated silica to minimize adsorption.
Temperature: The memory effect can be dramatically reduced by rising the temperature. For this, eliminating any cool spot in the gas-path is crucial. SUPER SESI transfer line is uniformly heated for this reason. The core is machined in a compact block to provide a uniform temperature. Since Sciex counter-flow is not heated, SUPER SESI for Sciex incorporates an extra heater to provide a uniform temperature distribution.
The temperature in the ionization region is limited by the boiling point of the electrospray liquid. As a result, this region is more susceptible to be contaminated. For this reason, minimizing the surface area in the ionization region is very important.Purity of the electro-spray liquid: the liquid can be contaminated during solution preparation, storage, or it can be contaminated if high purity grade solvents are not used. It is strongly advisable to use high purity grade water and formic acid. SUPER SESI is designed to minimize chances of contamination during spray handling.
Purity of the gas. If the gas supplied to the ionizer are contaminated, these contaminants will appear as background signals.
Fragmentation: fragmentation might occur if the electrospray is not properly formed and a corona discharge with a high energy region is formed instead. This can happen if the electrospray voltage is too high. To prevent the formation of coronas, SUPER SESI incorporates a dampener that drops the voltage if a corona discharge is formed. In addition, the narrow capillaries used in SUPER SESI require lower voltages that are not sufficient to initiate a discharge.
Cleaning: As obvious as it sounds, cleaning the ionizer improves the background levels.
How Super SESI is designed to minimize background levels:
Coating: the sample gas only encounters stainless steel coated with analytical grade passivated silica in its path to the ionization chamber. The clean gas inlet and all outlets are also coated to minimize contamination in the event that the gas accidentally flows backwards. Adsorption is dramatically reduced.
Improved sensitivity
Improved washing time
Improved memory effects
Electrode-less SESI: By eliminating the electrodes, this configuration greatly reduces the available surface onto which contamination can accumulate. Reducing the surface in the ionization region is particularly important because this region is particularly vulnerable to contamination:
The local temperature is limited by the boiling point of the electro-spray liquid.
The areas exposed to the ectrospray plume cannot be passivated because ions would otherwise accumulate onto the coated surfaces, thus destabilizing the electro-spray and changing the electrostatic configuration in an uncontrolled fashion.
Reversed flow: A small fraction of the sample flow is outputted through a secondary outlet located in the back of the electro-spray.
This causes the gas to flow backwards in the ionization chamber. As a result, contaminant released by the inner walls of the ionizer are dragged away from the ionization region.
These contaminants do not get ionized, and cannot contribute to the background levels of the SUPER SESI.
Cleaning flow: When no sample is introduced in SUPER SESI, the clean curtain gas fills the ionizer. Before this, the curtain gas is passed through a charcoal filter to ensure that no VOCs originating from the gas lines of the laboratory of the mass spectrometer enter the ionizer.
By default, SUPER SESI is cleaning itself in normal operation. Contaminants are washed by the clean gas.
This prevents room air from entering the ionizer, thus reducing the exposure.
Conventional cleaning: SUPER SESI can be easily disassembled for further cleaning, and incorporates a steam generator to steam-clean it. If, by any chance, SUPER SESI gets contaminated beyond what can be automatically self-cleaned, SUPER SESI can be cleaned the old way: with solvents, soap, or other cleaning solutions (this will depend on the specific contaminant that made it into the ionizer). Cleaning can be boring, but it is the best way to reduce the background levels. Once again, mum was right!
Steam-desorption cleaning: Steam-desorption is widely used to regenerate active adsorvents. In SUPER SESI, this mechanism is used to clean the internal surfaces without even needing the open the ionizer. The advantage of steam-desorption cleaning over conventional cleaning is that it is much faster.
Related products












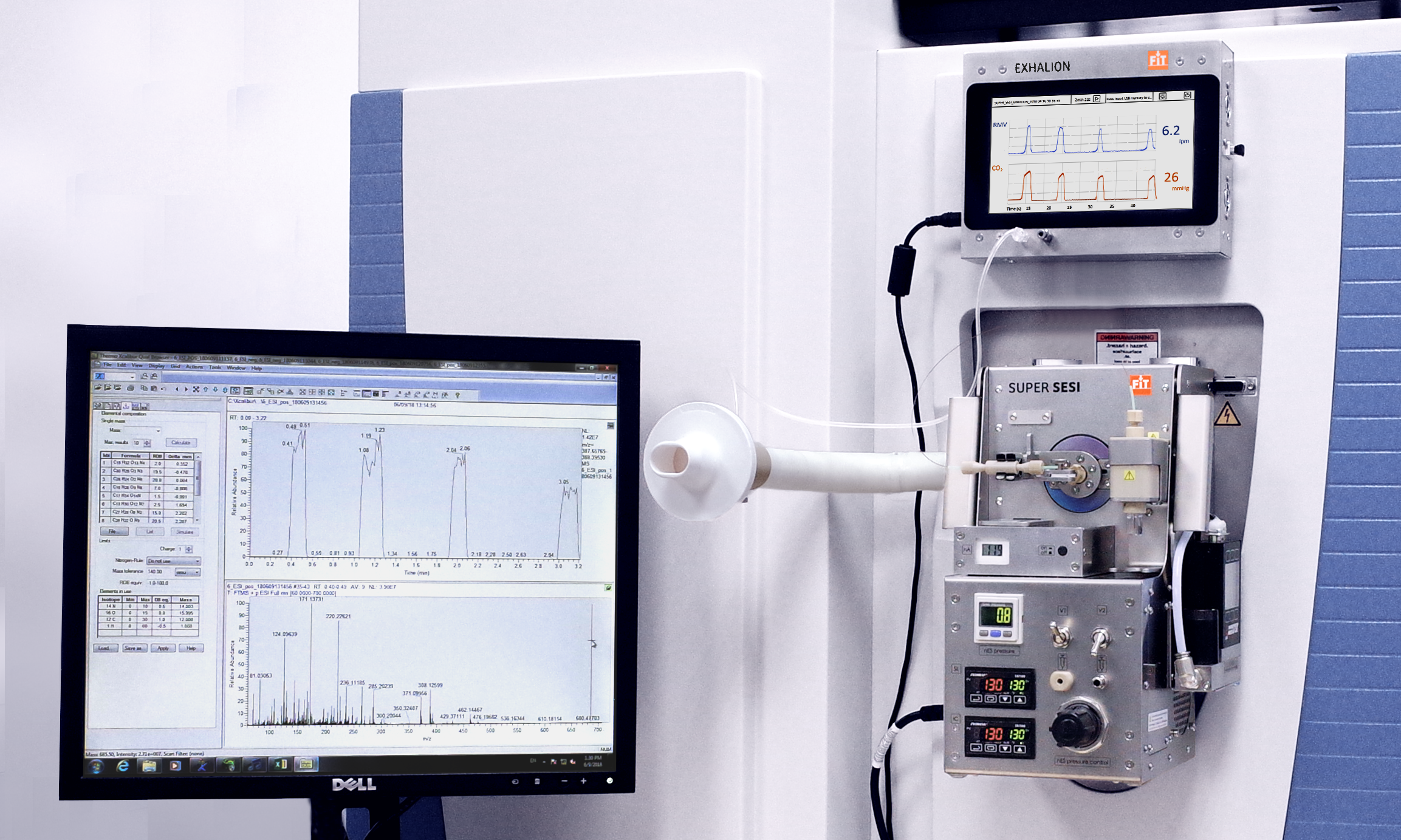




























Secondary electrospray ionization and tools like Super SESI allow us to safely detect large molecules in the gas phase, with an extremely low LoD.