
nano-Electrospray Ionization (ESI) for Proteomics
nano Electrospray ionization (ESI) produces ions from liquid samples. It is ideal for the analysis of large biomolecules, including proteins and lipids. A high voltage is applied to the liquid until coulombic repulsion overcomes surface tension. This produces tiny droplets highly charged. As droplets evaporate, they produce gas-phase ions. This results in extremely high ionization efficiency even when ion suppression would compromise regular spray.
How nano-electrospray works, from droplets to ions:
At the tip of the emitter, electrostatic stress equals surface tension of the liquid. this causes the conical meniscus (the Taylor cone). This results in a singularity at the tip of the cone where the electric fields is extremely intense.
A jet emerges from the tip of the cone. The jet then breaks up into a stream of tiny droplets.
First generation of droplets are very small, but not enough to shed ions. As solvent evaporates, the droplet charge is confined into a shrinking volume until surface tension can no longer hold the electrostatic stress and the droplet explodes.
Consecutive Coulombic fission events produce highly charged and tiny droplets (child droplets: 0.1% the volume and 25% the charge of parent droplet).
The evaporation/fission process rapidly produces extremely small droplets. When the nano-droplets reach the critical size (~10nm), ions are ejected. The ionization mechanism depends on the analyte and solvent composition.
1st generation droplets:
Reasons to aim for small droplets:
The sequence of evaporation droplet shrinkage and coulombic fission events leads to nano-droplets capable of shedding ions even if the spray produces large droplets. Considering that nano-electrospray comes with some added difficulty, why bother to produce nano- electrospray tiny droplets? Here are some reasons:
Improve ionization efficiency: As solvent evaporates, contaminants including salts accumulate in the resulting residue. The number of contaminants accompanying the molecule of interest is greatly reduced by starting with a smaller droplet.
Improve ion suppression susceptibility: The coulombic fissions transfers hydrophobic substances to the next generation more favorably because they stay in the surface of the droplet, which is more available during the fission event. This can lead to ion suppression of the more hydrophilic molecules. The number of fission events required to reach the ion shedding regime is reduced by starting with smaller droplets.
Improve sample utilization. Coulombic fission events leave low-charge droplets as a byproduct. These droplets do not produce ions and waste a lot of sample. They repel the ions of interests, saturate the ion optics and contaminate the ion path. This ‘zombie’ load is reduced by starting with smaller droplets.
nano-ESI dynamics and droplet size:
In normal nLC-MS proteomics workflows, the droplet size is about 200-500 nm *. This is well below the ID of the emitter, and it only requires about one fission step to reach the ion shedding scale (~10nm).
When the meniscus forms a stable jet, the droplet size depends on the liquid properties and the flow rate:
High conductivity, low electrical permittivity and low surface tension lead to smaller droplets.
Smaller flow rates also reduce the droplet size, but there is a minimum flow rate that can sustain a stable electrospray.
— 1st gen droplet size if defined by the dynamics of the meniscus —
The meniscus:
The quality of the signals depends on the size of the 1st generation nano-electrospray droplets, which is defined by the nano jet, which emerges from the electrospray meniscus, whose shape is determined by the emitter. The first step in this chain of cause and effect is at the electrospray meniscus. The key for a stable high performing nano-electrospray is a stable meniscus (forming a Taylor cone and a jet).
In short, to produce a stable signal, the meniscus has to be stable and as small as possible
Reasons to reduce the size of the meniscus:
Solvent evaporation is desirable at the droplets but too much evaporation at the meniscus increases the concentration of contaminants reaching the jet. This enhances ion suppression effects and changes the properties of the liquid, affecting the droplet size, and the optimum the flow and voltage conditions. Smaller meniscus means less solvent evaporation.
Ion evaporation is desirable at the droplets but, at the vicinity of the nano-jet, this further ionize the gas because of high energy collisions induced by the strong electric fields. These gas ions reduce the droplets charge and hence their ionization efficiency. Ion evaporation is enhanced by solvent evaporation. Smaller meniscus means less ion evaporation.
Corona discharges form in the gas surrounding the meniscus when the voltage is too high. Ions formed at the discharge are attracted to the droplets and reduce their net charge. The voltage required to form an electrospray depends on the meniscus size. Small meniscus means low voltages an no corona discharges.
Ion transport: Smaller meniscus produce weaker electric fields pushing the ions forward. For this reason, nano-electrospray must be located very close to the MS inlet. The smaller the meniscus, the smaller the emitter to MS inlet distance.
How the base of the meniscus is formed:
The meniscus is anchored at the point where the meniscus cone meets the emitter at the contact angle.
In practice, the contact angle is very low because the emitter surface rapidly becomes wettable due to the harsh environment. This means that the anchorage line forms at the point where the meniscus is tangent to the emitter.
The meniscus cone angle (β) varies with the spray voltage, flow rate, and the liquid properties. The meniscus size is defined by its angle and the emitter outer geometry, not the emitter Inner Diameter (ID)!
Spray stability:
Provided that the meniscus size is well defined and sufficiently small, the stability of the spray is mainly affected by:
The spray voltage: There is a range of voltages that produce stable sprays, which depends on the meniscus size. In nano-ESI proteomics workflows, this is 1.7 to 2.5 KV.
Flow rate. The theoretical minimum jet flow for proteomics workflows is below 1nL/min, but the evaporated flow is 10-20 nL/min for online 10-20μm ID emitters. In practice, the flow rate is limited by solvent evaporation.
Other common sources of instability include:
Lateral wetting instabilities: The meniscus points sideways.
Longitudinal wetting instabilities: unstable meniscus size changes with moving anchorage line.
Clogging: residues accumulate at the tip.
Bubbles: as the liquid flows through the column and the emitter, dissolved gases tend to precipitate. As bubbles expand, they distort the flow rate reaching the meniscus, its geometry, and break the electric conductivity.
The base of the meniscus:
The emitter defines the base of the meniscus, what is the optimum geometry?
Very acute angle: stable meniscus anchorage requires the emitter cone (α) to be smaller than the meniscus cone angle (β). Sharper emitters can accommodate a wider range cone angles.
Well-defined edge: with this abrupt geometry, the meniscus sits at the same anchorage diameter regardless of the angle (β). In contrast, the meniscus size varies with β in rounded tips. This edge eliminates a source of variability, thus improving the consistency of the results.
Emitter Inner Diameter: the emitter ID capillary is a just tiny pipe that feeds the meniscus as its content gets sprayed. For robustness, the ID should be as large as possible to prevent clogging.
Surface chemistry: liquid-emitter interaction
Molecules that adhere to the binding sites of the glass surface are not ionized. This reduces ionization efficiency. The good news is each binding site can hold only one molecule. In practice the glass has a limited number of binding sites. In two runs, most binding sites are saturated and the emitter reaches top performance.
to minimize internal losses, the inner surface of the emitter has to be as inert as possible to minimize chemical interactions between the molecules passing through the emitter and the emitter.
Hydrophobic emitters stabilize the meniscus at the inner diameter of the emitter. For the same emitter geometry, this results in a smaller meniscus, with a more stable anchorage. This produces less evaporation, lower voltages, better ionization efficiency, and a more consistent spray.
Our solutions:
Electrospray and nano-electrospray ionization are a key component of all nLC-MS workflows. The performances and reliability of sampling, LC, and MS technologies have evolved impressively in the last decade. Yet, electrospray emitters have largely remained the same. Repeatability and stability issues are a normal thing in most proteomics labs.
We are on a mission to make nano-electrospray reliable for proteomic workflows. nESI provides the best-known performance, but requires the user to be constantly on top of little (yet crucial) details. Our approach to this is to pay attention to these details in the design and production process so that the spray just works.
For that, we created our emitters “The Sharp Singularity” and Simple Link.
nESI Emitters, the Sharp Singularity (Regular or LOTUS)
The Sharp Singularity Emitters are mechanically sharpened under the microscope. This allows us to control the geometry of the tip, which is optimized for performance, robustness and repeatability.
All Sharp Singularity emitters are delivered with a quality control report with microscopic photos, so that you have a clear view of the geometry you are using.
Simple Link - Uno
Simple Link-Uno provides voltage to the electrospray and connects the column and emitter with an accessible Zero Dead Volume fitting, reducing complexity and chances of error.
ELECTROSPRAY ACADEMY:
The purpose of this section is to help you get better electrospray more consistently. We add new content regularly to cover electrospray related topics in a didactic way, so make sure to check!
Playing with ions. Our electrospray and ionization educational Youtube channel! Here we explain in an easy way the complex world of the Electrospray :)
KNOW YOUR EMITTER series: educational series posted on our blog will help you to understand better all the details related with emitters.
Downloadable content related with emitters and electrospray. Dowload now and read later!
Download this Application note that explains how to set the voltage to expand the life of your LOTUS emitters
Download this White Paper explains nano-Electrospray ionization in simple terms
this Tech Note studies the run-to-run variability of a standard proteomics workflow and shows the repeatability and the stability of the signals and the protein group identifications.
Download, use the spreadsheet to estimate key parameters of your nano-electrospray






















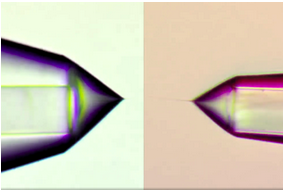





The new LOTUS emitters improve protein ID and reproducibility with a hydrophobic coating that stabilizes the meniscus.
This application note shows how to extend the lifespan of your LOTUS emitters.